Mẹo Hướng dẫn What is the difference between single and double displacement reactions 2022
Dương Minh Dũng đang tìm kiếm từ khóa What is the difference between single and double displacement reactions được Update vào lúc : 2022-11-19 16:02:09 . Với phương châm chia sẻ Kinh Nghiệm về trong nội dung bài viết một cách Chi Tiết 2022. Nếu sau khi tham khảo tài liệu vẫn ko hiểu thì hoàn toàn có thể lại Comments ở cuối bài để Tác giả lý giải và hướng dẫn lại nha.What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Nội dung chính Show- What is a replacement reaction?What are single replacement reactions?What are double replacement reactions?Suggested ReadingWhat is the difference between displacement and double displacement reaction with example?How would you compare a single displacement reaction to a double displacement reaction?
In a displacement reaction, a more reactive element replaces a less reactive element from a compound.
For Example: CuSo4 (aq) + Zn (s) → ZnSO4 (aq) + Cu (s)
In a double displacement reaction, two atoms or a group of atoms switch places to
form new compounds.
For Example: Na2SO4 (aq) + BaCl2 (aq) → BaSO4 (s) + 2NaCl (aq)
- A displacement reaction is the one when a more reactive substance displaces a less reactive one from its salt solution whereas a double displacement reaction is the one where a mutual exchange of ions happens between two compounds.In a
displacement reaction only a single displacement takes place whereas in the double displacement reaction, as the name suggests two displacement takes place between the molecules.Example:Displacement reaction
Mg+2HCl→MgCl2+H2
- Double displacement reaction
2KBr+BaI2→2KI+BaBr2
Displacement Reaction Double Displacement Reaction Displacement reaction is a chemical reaction in which displacement of a metal from its solution takes place by the other metal that is more reactive than the metal being displaced. Double displacement reaction is a chemical reaction in which exchange of ions between both the reactants takes place. Example:
Zn + CuSO4 → ZnSO4 + Cu
Here, Zinc (Zn), which is more reactive than Cu, displaces copper from its solution. Example:
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
Here, both Na2SO4 and BaCl2 exchange their ions.
Concept: Types of Chemical Change or Chemical Reaction - Single Displacement Reactions
Is there an error in this question or solution?
In single replacement reactions, one element replaces another in a chemical compound. In double replacement reactions, the swapping of elements takes place.
Imagine a hypothetical situation where a kid who loves ice cream is going out with his two friends. Initially, he is holding hands with Friend A, but Friend B soon joins then and offers his ice cream to the child. Whose hand do you think he will want to hold then? Obviously, Friend B! Kids are like that. They easily drift towards people who offer them the things they love. Funnily, chemistry also đơn hàng with these types of interactions, which are called reactions.

A chemical reaction in process (Photo Credit : Shaiith/Shutterstock)
A replacement reaction is a type of chemical reaction in which one element replaces another in a compound. This can either be in the form of a single replacement reaction or a double replacement reaction. In a single replacement reaction, one of the reactants is more reactive than the other, which results in the formation of a product that is more stable. In double replacement reactions, the elements get replaced in both the reacting compounds. Double replacement reactions also result in the formation of a solid product, which is called a precipitate.
But how do these reactions take place? Let’s take a look!
Recommended Video for you:
Which Is The Most Reactive Element In The Periodic Table?
If you wish to buy/license this video, please write to us .
What is a replacement reaction?
Before diving into the subtypes, let’s explore the basics of this chemistry principle. Have you ever seen a rusted iron rod? This type of object is a prime example of a chemical reaction. A process in which one or more substances are converted into one or more different substances is called a chemical reaction. The substances that undergo a change are referred to as reactants, while those being formed are called products. In the case of rusting, our reactants are represented by iron and water vapor in the air. When these two react, they form rust, which is referred to as our product.

The Chemistry of Rusting (Photo Credit : Nasky/Shutterstock)
However, to classify reactions and make things a bit easier, we have broken this down into specific categories. In replacement reactions, more reactive elements replace the less reactive elements of a compound to form a stable product. These replacement reactions are also known as displacement reactions.
What are single replacement reactions?
Now that you know what replacement reactions are, let’s take a look one of its subtypes. Consider the following reaction: A + BC —— B + AC
Here, A is an element, and BC is a compound, but A is more reactive than the compound BC. Therefore, when they undergo a chemical reaction, A will replace B, as it is more reactive, to form the compound AC. B is given out in either an elemental or ionic form. Only a single compound undergoes replacement in this reaction, thus the name “single replacement”.
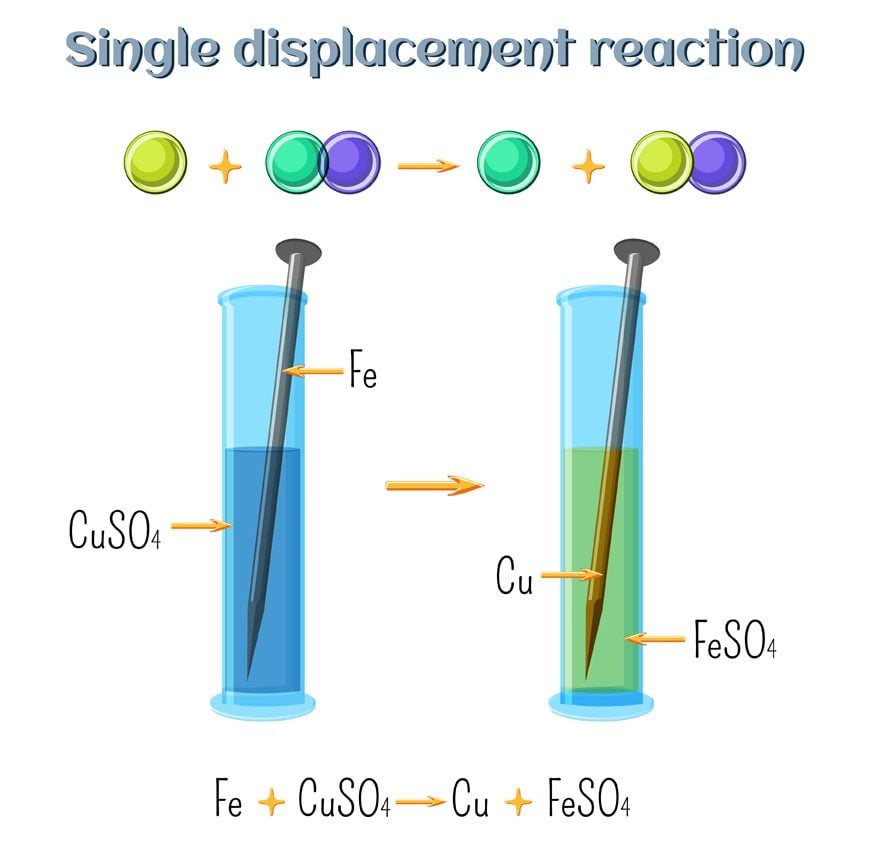
A diagrammatic representation of Single Replacement Reaction (Photo Credit : Inna Bigun/Shutterstock)
To understand this better, let’s consider the following examples:
Zn + CuCl2 —— ZnCl2 + Cu
This reaction easily fits in with the above explanation. Zinc is more reactive than Copper. Thus, when it is added in a solution of Copper Chloride, it replaces Copper and forms Zinc Chloride. Copper is given out in the ionic form. This reaction is also an excellent example of cationic replacement. We say this because the replacing ion has a positive charge, i.e., 2+. As such, it is a cation.
Br2 + 2KI —— 2KBr + I2
Similarly, when Bromine is added to a solution of Potassium Iodide, it replaces the position of Iodine in the compound. As a result, Potassium Bromide is formed, whereas a molecule of Iodine is given out. This reaction takes place because Bromine is more reactive than Iodine, which is why it replaces it. Because the replacement ions have a negative charge, this reaction is an example of anionic replacement.
What are double replacement reactions?
The easiest way to understand a double replacement reaction is by remembering the term “swapping partners”. In double replacement, the elements get replaced in both the reacting compounds. Here, parts of two ionic compounds are exchanged, making two new compounds. Let’s consider the following reaction: AB + CD —— AC + BD
Here, the elements in the reacting compounds exchange their partners. In the product, A combines with C, while B combines with D. Because the replacement occurs in two places, it is referred to as a double replacement. Double replacement reactions are also called metathesis or double displacement reactions. Usually, in these reactions, when combining aqueous solutions, a solid product is also formed. This product is referred to as a precipitate, and the reaction is called a precipitation reaction.
For example, take a look this reaction:
AgNO3 (aq) + NaCl (aq) —— AgCl (s) + NaNO3 (aq)
When an aqueous solution of Silver Nitrate is mixed with an aqueous solution of Sodium Chloride, it results in the formation of a Sodium Nitrate solution, while Silver Chloride precipitates out. One can easily see that elements have been replaced in both the reacting compounds.
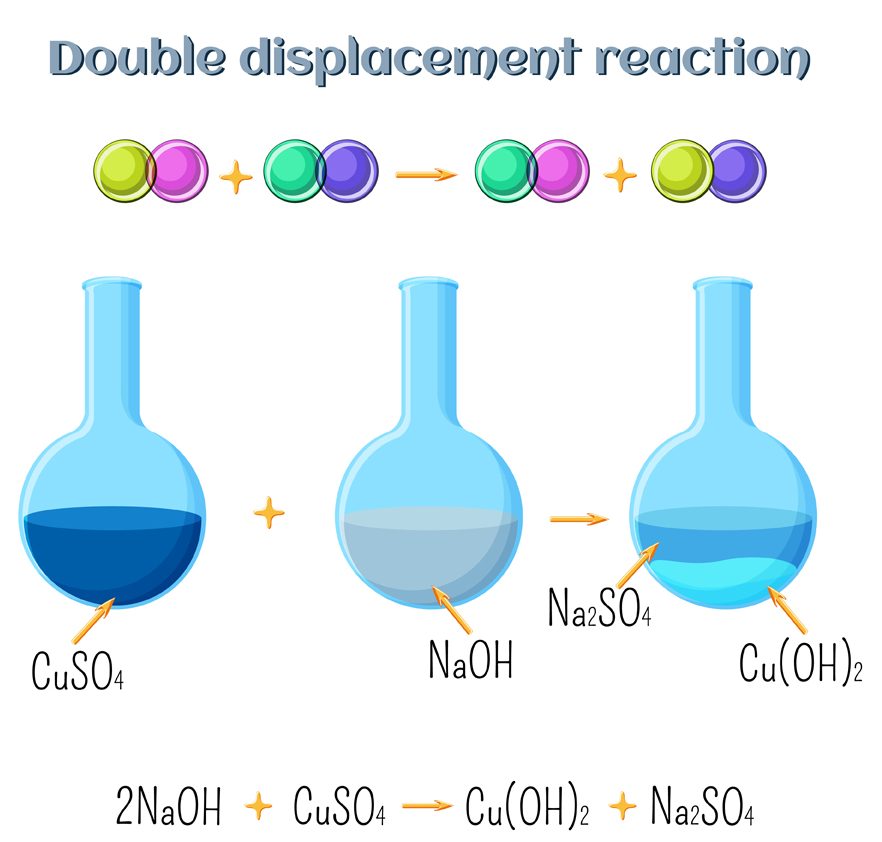
A diagrammatic representation of Double Replacement Reaction (Photo Credit : Inna Bigun/Shutterstock)
To summarize, in single replacement reactions, the more reactive element takes the place of a less reactive compound. On the other hand, in double replacement reactions, both of the reacting compounds swap partners and end up forming entirely new compounds!
Suggested Reading
Silent Witnesses: The Often Gruesome But Always Fascinating History of Forensic Science
Molecules: The Elements and the Architecture of Everything
Salt: A World History
Was this article helpful?
YesNo
Help
us make this article better
Scientific discovery can be unexpected and full of chance surprises. Take your own here and learn something new and perhaps surprising!
What is the difference between displacement and double displacement reaction with example?
Answer: (a) Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Both metals and non-metals take part in displacement reaction. Double displacement reaction takes place when two atoms or group of atoms switch places forms new compound.How would you compare a single displacement reaction to a double displacement reaction?
The key difference between single displacement and double displacement reaction is that, in single displacement reactions, one chemical species replaces a part of another chemical species whereas, in double displacement reactions, exchange of two ionic species between two molecules occur. Tải thêm tài liệu liên quan đến nội dung bài viết What is the difference between single and double displacement reactions